Glial cells in the peripheral nervous system (PNS) play vital supportive roles, encasing neurons to provide insulation, metabolic aid, and protection, distinct from their CNS counterparts. This diagram focuses on a unipolar peripheral ganglionic neuron, illustrating how satellite cells and Schwann cells interact with the cell body and axon, ensuring efficient signal transmission and repair in sensory and autonomic pathways. These cells highlight the PNS’s regenerative capacity, contrasting with the CNS, and underscore their importance in maintaining neural integrity across nerves extending from the spinal cord and brain to peripheral tissues.
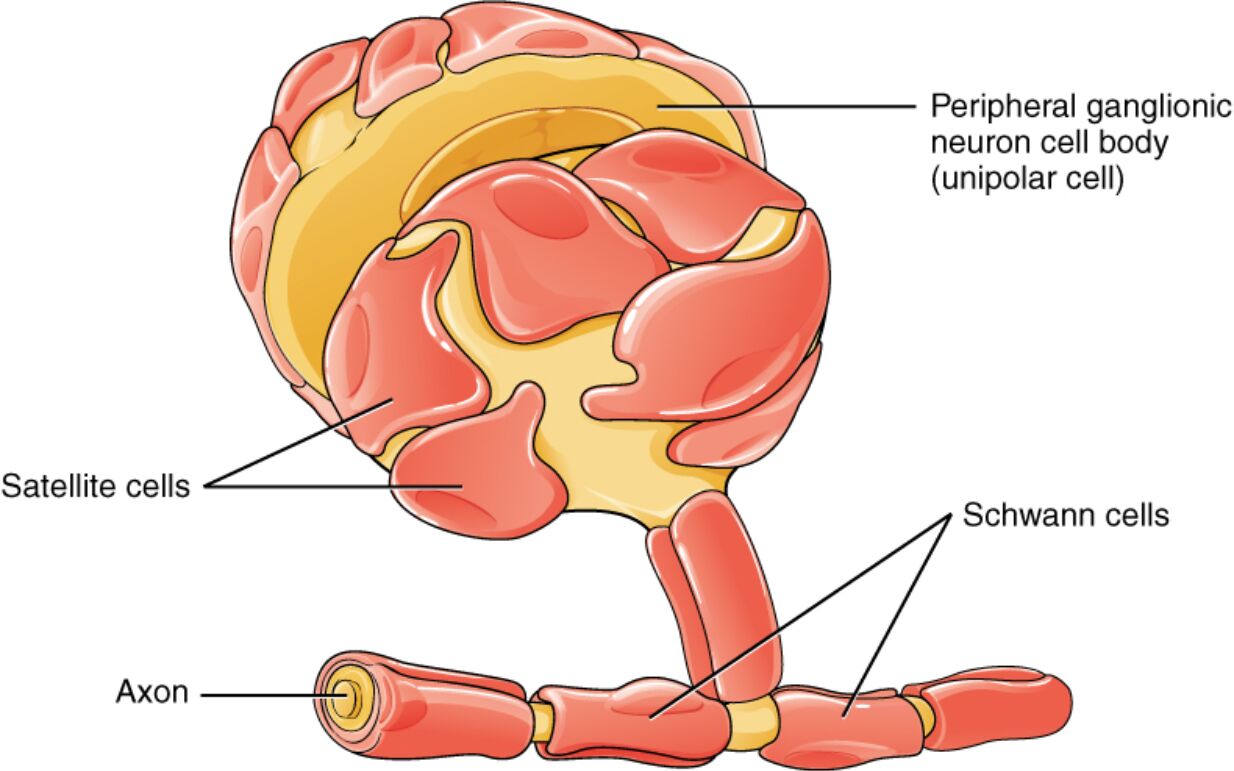
Labeled Components in the PNS
Peripheral ganglionic neuron cell body (unipolar cell)
The peripheral ganglionic neuron cell body, depicted as a rounded, yellow structure, houses the nucleus and organelles of a unipolar neuron, where a single process bifurcates into central and peripheral branches for sensory signal relay. Located in ganglia like dorsal root or autonomic types, it integrates inputs without dendrites, relying on the branching process for reception and transmission to the CNS.
Satellite cells
Satellite cells appear as pink, capsule-like layers surrounding the neuron cell body, forming a protective sheath within peripheral ganglia. These glial cells regulate the extracellular environment by controlling ion and nutrient exchange, and they support neuronal metabolism while isolating individual cell bodies to prevent cross-talk in densely packed ganglia.
Schwann cells
Schwann cells are shown as segmented, red coverings along the axon, each wrapping around a portion to form myelin sheaths or Remak bundles in unmyelinated fibers. In myelinated axons, they produce lipid-rich myelin to accelerate nerve conduction, while in injury scenarios, they dedifferentiate to guide axonal regrowth and clear debris via phagocytosis.
Axon
The axon is illustrated as a long, yellow extension emerging from the cell body, segmented by Schwann cell wrappings, carrying electrical impulses away from the soma toward targets like muscles or glands. In the PNS, axons can regenerate post-injury, supported by glial cells, with myelinated ones featuring nodes of Ranvier for saltatory conduction at speeds up to 100 m/s.
Detailed Anatomy of PNS Glial Cells
The structure of PNS glial cells is adapted for dynamic interactions in a system prone to mechanical stress and regeneration. Anatomical features emphasize their enveloping and insulating properties.
- Satellite cells form multilayered envelopes around soma in sensory and autonomic ganglia, expressing glutamine synthetase for neurotransmitter precursor synthesis.
- Each satellite cell contacts one neuron but shares boundaries with others, creating a perineuronal space regulated by tight junctions.
- Schwann cells in myelinating forms spiral their plasma membrane up to 100 times around axons, compacting into myelin with major dense lines from PLP proteins.
- For unmyelinated axons, Schwann cells bundle multiple fibers in Remak processes, providing loose ensheathment without compact myelin.
- The axon itself varies in diameter from 0.2 μm in C-fibers to 20 μm in Aα-fibers, influencing myelination patterns and conduction velocity.
Physiological Functions in the PNS
Glial cells in the PNS actively contribute to neural signaling and homeostasis. Their functions extend to metabolic support and immune surveillance.
- Satellite cells buffer potassium ions during neuronal activity, preventing hyperexcitability via Kir4.1 channels, and supply trophic factors like NGF for neuron survival.
- They also phagocytose debris in ganglia post-injury, aiding in the PNS’s superior regenerative environment compared to the CNS.
- Schwann cells facilitate saltatory conduction by clustering sodium channels at nodes, reducing energy use through limited ion exchange.
- In development, Schwann cells migrate along axons guided by neuregulin-1 from ErbB receptors, sorting fibers for myelination based on diameter thresholds.
- Axons rely on glial-derived cholesterol for myelin synthesis, as neurons lack key enzymes like HMG-CoA reductase in adulthood.
Glial-Neuronal Interactions in the PNS
Interactions between glia and neurons ensure coordinated function and response to stimuli. These partnerships are crucial for peripheral sensation and autonomic control.
- Satellite cells communicate with neuron somata via gap junctions, allowing direct metabolite transfer, and modulate pain signaling in conditions like neuropathic pain.
- They express receptors for ATP and Substance P, released from active neurons, to fine-tune ganglionic excitability.
- Schwann cells form basal lamina tubes post-axotomy, providing a scaffold rich in laminin for regenerating axons to follow.
- During Wallerian degeneration, Schwann cells upregulate p75NTR receptors to promote macrophage recruitment for myelin clearance.
- Axonal transport of mitochondria and vesicles is supported by glial energy provision, preventing degeneration in long peripheral nerves.
Developmental Aspects of PNS Glia
Development of PNS glial cells involves neural crest origins and maturation signals. These processes shape the mature system’s regenerative potential.
- Satellite cells and Schwann cells derive from Schwann cell precursors (SCPs) migrating from neural crest, differentiating under Sox10 transcription factor control.
- Boundary cap cells at nerve roots give rise to some satellite populations, ensuring glial coverage at CNS-PNS transitions.
- Myelination by Schwann cells begins postnatally, timed by axonal Krox-20 expression, with myelin thickness optimized by g-ratio of about 0.6-0.7.
- In embryonic stages, immature Schwann cells promote neuronal survival via BDNF secretion, pruning excess axons through controlled apoptosis.
- Genetic mutations in PMP22 can disrupt this development, leading to dysmyelination as seen in hereditary neuropathies.
Research Techniques for Studying PNS Glia
Modern methods illuminate glial roles at cellular and molecular levels. Techniques focus on visualization and functional assays.
- Electron microscopy reveals ultrastructure, showing Schwann cell cytoplasm channels (Schmidt-Lanterman incisures) for nutrient diffusion in myelin.
- Live imaging with teased nerve preparations tracks Schwann cell dedifferentiation post-injury using fluorescent reporters like NG2.
- Patch-clamp electrophysiology measures ion currents in satellite cells, identifying voltage-gated channels involved in homeostasis.
- Single-cell RNA sequencing profiles glial heterogeneity, distinguishing myelinating from non-myelinating Schwann subtypes by genes like MBP versus NCAM.
- Animal models like sciatic nerve crush demonstrate glial-mediated regeneration, quantified by axon counting and gait analysis.
Clinical Implications and Pathologies
Glial dysfunction in the PNS contributes to various disorders, informing diagnostic and therapeutic approaches. Pathologies often involve demyelination or inflammation.
- Charcot-Marie-Tooth disease arises from Schwann cell gene mutations (e.g., MPZ), causing progressive weakness due to impaired myelin stability.
- Guillain-Barré syndrome features autoimmune attack on Schwann myelin, leading to acute paralysis treatable with IVIG or plasmapheresis.
- Satellite cell hyperplasia in neurofibromatosis type 1 forms benign tumors, compressing neurons and causing pain or sensory loss.
- Traumatic nerve injuries benefit from glial support, with surgical interventions like autografts leveraging endogenous Schwann migration.
- Chronic pain syndromes involve sensitized satellite cells releasing pro-inflammatory cytokines like IL-6, amplifying nociceptor firing.
In summary, the depicted satellite and Schwann cells exemplify the PNS’s glial framework, essential for neuron protection, efficient conduction, and robust repair mechanisms. Exploring their anatomy and physiology not only enhances understanding of peripheral neural operations but also drives innovations in treating neuropathies and injuries, contributing to improved neurological care.

