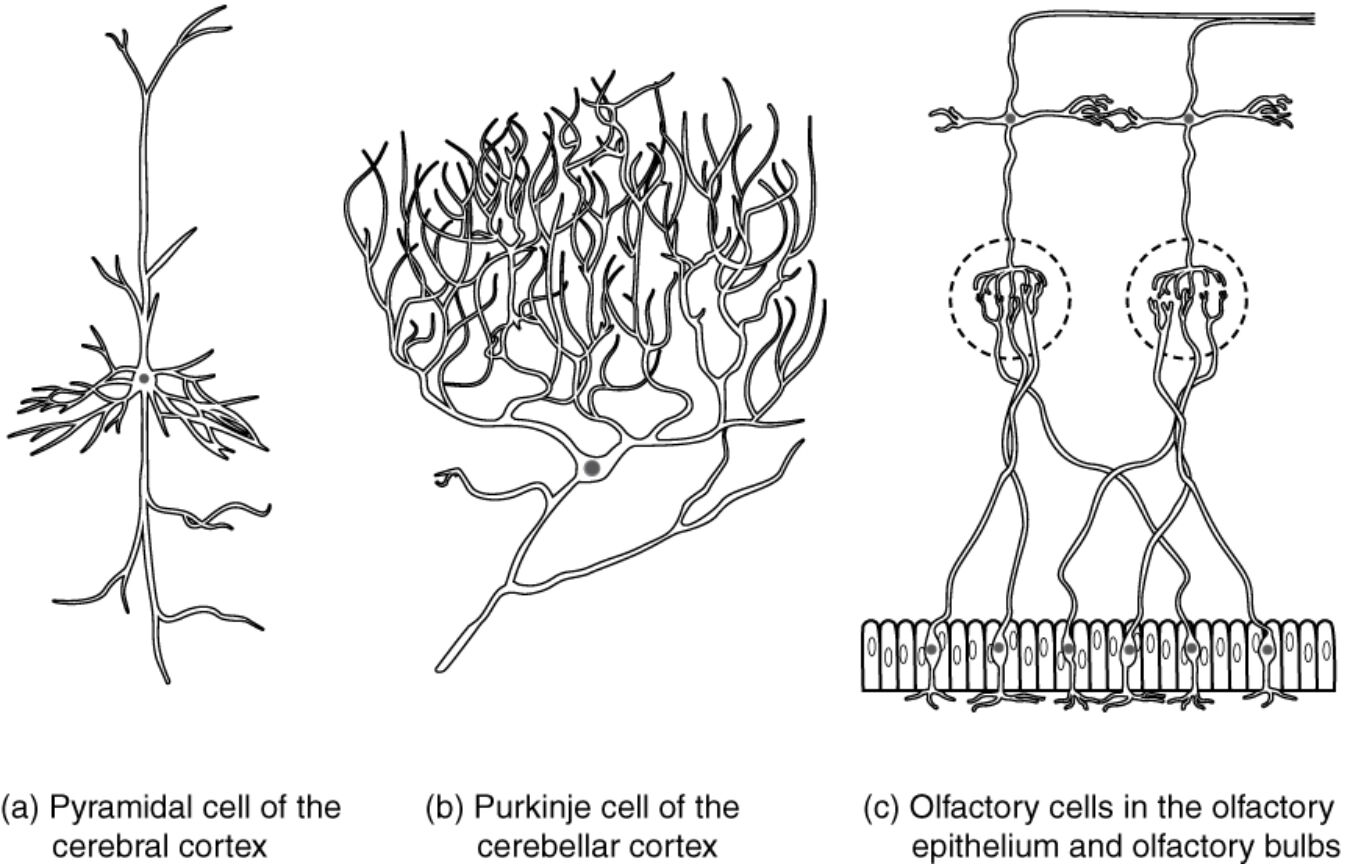
Neurons display remarkable diversity in form and function, extending beyond basic classifications to include specialized types adapted for specific roles in cognition, coordination, and sensation. This diagram illustrates three distinctive examples: the pyramidal cell of the cerebral cortex, the Purkinje cell of the cerebellar cortex, and olfactory cells in the olfactory epithelium and bulbs, each named based on shape, discoverer, or functional association. These neurons highlight how morphological adaptations enhance efficiency in neural processing, from integrating vast inputs in the brain to detecting odors in the nasal cavity, providing insights into the intricate architecture of the nervous system.
Labeled Neuron Types
Pyramidal cell of the cerebral cortex
The pyramidal cell features a pyramid-shaped cell body with a prominent apical dendrite extending upward and basal dendrites spreading laterally, classifying it as a multipolar neuron essential for cortical processing. These cells form layered structures in the cerebral cortex, projecting long axons to distant brain regions or the spinal cord to facilitate higher-order functions like decision-making and voluntary movement.
Purkinje cell of the cerebellar cortex
The Purkinje cell exhibits a highly elaborate dendritic arbor that fans out in a single plane, resembling a trellis, with a single axon exiting the base to inhibit deep cerebellar nuclei. Named after anatomist Jan Evangelista Purkinje, these large multipolar neurons integrate inputs from thousands of parallel fibers and climbing fibers, playing a crucial role in fine-tuning motor control and learning.
Recommended Study Resource
Gray's Anatomy: The Anatomical Basis of Clinical Practice
Enhance your anatomical knowledge with Gray's Anatomy: The Anatomical Basis of Clinical Practice. This authoritative text offers in-depth insights and illustrations, perfect for medical students and practitioners aiming for clinical excellence.
At AnatomyNote.com, we offer free resources on anatomy, pathology, and pediatric medicine for medical students and professionals. Purchasing through our Amazon links, like Gray's Anatomy, supports our server costs and content creation at no additional cost to you.
Disclosure: As an Amazon Associate, we earn a commission from qualifying purchases.
Disclosure: As an Amazon Associate, we earn a commission from qualifying purchases at no extra cost to you.
Olfactory cells in the olfactory epithelium and olfactory bulbs
Olfactory cells, or olfactory receptor neurons, are bipolar in nature with dendrites extending to the nasal mucosa for odorant detection and axons bundling into glomeruli in the olfactory bulbs. These neurons belong to the sensory functional group, continuously regenerating to maintain smell perception, and synapse with mitral cells in the bulbs to relay olfactory information to higher brain areas.
Detailed Anatomy of Specialized Neurons
Each neuron type showcases unique structural features tailored to its environment and tasks. Anatomical details reveal evolutionary optimizations for signal handling.
- Pyramidal cells in layers II/III and V of the cerebral cortex have spiny dendrites that increase synaptic surface area, allowing convergence of excitatory inputs from thalamocortical afferents.
- The apical tuft in pyramidal neurons branches extensively in layer I, receiving feedback from association cortices to modulate contextual information.
- Purkinje cells possess up to 200,000 spines on their dendrites, each forming synapses with parallel fibers from granule cells, enabling massive parallel processing.
- Their soma contains abundant rough endoplasmic reticulum for synthesizing GABA, the primary inhibitory neurotransmitter they release.
- Olfactory receptor neurons express specific G-protein-coupled receptors (like OR1A1 for certain odors), with cilia on dendrites amplifying chemical signals via cyclic AMP pathways.
- In the olfactory bulbs, axons form synaptic glomeruli where convergence ratios of 1000:1 enhance signal-to-noise ratios for odor discrimination.
Physiological Functions and Mechanisms
Physiological operations in these neurons involve electrochemical signaling finely tuned by their morphologies. Functions range from integration to transduction, supporting complex behaviors.
- Pyramidal cells generate action potentials that propagate down corticospinal tracts, with back-propagating potentials in dendrites facilitating synaptic plasticity via NMDA receptors.
- They release glutamate excitatorily, contributing to long-term potentiation (LTP) in hippocampal CA1 pyramids for memory formation.
- Purkinje cells fire at high rates (up to 200 Hz) in simple spikes from parallel fiber inputs, while complex spikes from climbing fibers trigger calcium influx for motor adaptation.
- Inhibitory output modulates cerebellar output, refining movements through error correction in vestibulo-ocular reflexes.
- Olfactory neurons transduce odors into graded potentials, with each cell expressing one receptor type from a repertoire of about 400 in humans, ensuring diverse odor coding.
- Depolarization leads to neurotransmitter release in bulbs, where lateral inhibition via periglomerular cells sharpens scent patterns.
Roles in Neural Circuits and Systems
These neurons integrate into larger networks, influencing system-level operations. Circuitry emphasizes connectivity and specialization.
Anatomy Flash Cards
Master anatomy with detailed, exam-ready flash cards.
AnatomyNote.com offers free anatomy and pathology resources. Your purchase of Anatomy Flash Cards supports our site at no extra cost.
As an Amazon Associate, we earn from qualifying purchases.
- Pyramidal cells form columnar organizations in the neocortex, with layer V cells projecting to subcortical structures like the basal ganglia for action selection.
- Recurrent connections among pyramids enable oscillatory rhythms, such as gamma waves associated with attention and perception.
- Purkinje cells receive sole excitatory input from one climbing fiber per cell, originating from inferior olivary nuclei, for precise timing in motor learning.
- They synapse onto deep nuclei, which in turn project to thalamic relays, closing loops with cerebral motor areas.
- Olfactory receptor neurons project ipsilaterally to bulbs, where mitral and tufted cells relay to piriform cortex for conscious smell and amygdala for emotional associations.
- Bulbar circuits include granule cells for feedback inhibition, adapting to odor intensity changes.
Developmental and Histological Aspects
Development shapes these neurons through genetic and activity-dependent processes. Histological techniques have historically revealed their intricacies.
- Pyramidal cells differentiate from radial glia progenitors, migrating along glial guides to form laminar patterns guided by Reelin signaling.
- Dendritic growth involves BDNF neurotrophins, pruning excess branches postnatally based on synaptic activity.
- Purkinje cells arise from ventricular zone precursors, with dendritic elaboration requiring agrin from afferents for spine formation.
- Golgi impregnation, used by Ramón y Cajal, first visualized their planar dendrites, highlighting compartmentalization.
- Olfactory neurons derive from placodal ectoderm, with ongoing neurogenesis from basal cells involving Notch signaling for progenitor maintenance.
- Axonal guidance to specific glomeruli relies on odorant receptor expression dictating targeting via ephrin gradients.
Research Methods and Imaging Techniques
Advanced tools elucidate structure-function relationships in these neurons. Imaging and electrophysiology provide dynamic insights.
- Two-photon calcium imaging tracks dendritic spikes in pyramidal cells during behavior, revealing compartment-specific computations.
- Electron microscopy reconstructs synaptic connectomes, showing asymmetric synapses in cortical pyramids.
- Patch-clamp recordings from Purkinje dendrites measure local currents, identifying P/Q-type calcium channels critical for spike bursts.
- Optogenetic stimulation of climbing fibers dissects their role in cerebellar plasticity.
- In vivo multiphoton microscopy visualizes olfactory neuron turnover, correlating receptor expression with functional maps via intrinsic signal imaging.
- Single-cell RNA sequencing profiles transcriptomes, identifying subtype diversity in bulb interneurons.
Clinical Relevance and Pathological Considerations
While the diagram focuses on normal anatomy, understanding these neurons informs pathology. Disruptions lead to neurological deficits.
- Pyramidal cell loss in Alzheimer’s involves tau tangles, impairing axonal transport and cortical connectivity.
- Excitotoxicity in stroke damages pyramids, causing hemiparesis via disrupted descending pathways.
- Purkinje cell degeneration in spinocerebellar ataxias, like SCA1, results from polyglutamine expansions, leading to gait instability.
- Autoimmune attacks in paraneoplastic syndromes target Purkinje antigens, manifesting as cerebellar ataxia.
- Olfactory neuron dysfunction in anosmia, as in COVID-19, stems from viral entry via ACE2 receptors on supporting cells.
- Congenital hyposmia links to Kallmann syndrome, with failed migration of olfactory precursors alongside gonadotropin-releasing hormone neurons.
In summary, the illustrated pyramidal, Purkinje, and olfactory neurons exemplify the specialized adaptations that underpin neural diversity, enabling precise functions from thought to balance and scent detection. Delving into their anatomy and physiology not only enriches knowledge of brain organization but also guides therapeutic strategies for disorders affecting these critical cells, advancing neuroscience toward better health outcomes.




